How antibiotic overuse in human medicine impacts beef producers
While animal agriculture is the focus of antibiotic-resistant bacteria in people, the human use of antibiotics must be considered as well. Fourth of a six-part series.
June 23, 2017
“If we don’t address the problem of antibiotic resistance, we may lose quick and reliable treatment of infections that have been a manageable problem in the United States since the 1940s. Drug choices for the treatment of common infections will become increasingly limited, and in some cases, nonexistent.”
That’s a statement from the U.S. Centers for Disease Control and Prevention (CDC) in 1999. Dr. Kurt Stevenson shared it at last year’s Antibiotics Symposium hosted by the National Institute for Animal Agriculture (NIAA). He is an infectious disease physician in the division of infectious diseases at Ohio State University’s Wexner Medical Center.
“I can say very clearly that we’re at that point,” Stevenson explained. “We have patients now who have drug-resistant pathogens for which there is either one drug — or no drug — available to treat them.”
“We see this on a fairly regular basis in our medical center,” he said. “We have a number of patients who have very limited choices, and as a consequence, we have to use very expensive drugs and drugs that tend to have higher toxicity.”
Ever since Alexander Fleming rediscovered penicillin in 1928, followed by wide-scale production in the 1940s, health care providers worldwide have continued to grow increasingly dependent on nature’s wonder drugs to combat bacterial infection. Doctors in the U.S. are no exception.
Health care providers in the U.S. prescribed 266.1 million antibiotic prescriptions in 2014 — equivalent to 835 antibiotic prescriptions per 1,000 people, according to CDC. That’s equivalent to five prescriptions written for every six people each year.
Today, the United States is the third-largest consumer of antibiotics in human medicine in the world, according to Dr. Laura Kahn, a physician and research scholar with Princeton University’s program on science and global security at the Woodrow Wilson School of Public and International Affairs. Only India and China consume more.
“That’s total use,” Kahn emphasized. “The countries with the highest per capita use of antibiotics, for whatever reason, are Australia and New Zealand.”
One Health Initiative
Kahn is also co-founder of the One Health Initiative.
“One Health is very simply the concept that human, animal and environmental health are linked,” Kahn told participants at last year’s annual convention of the Texas Cattle Feeders Association (TCFA). “And because they are linked, complex subjects such as antimicrobial resistance must be examined in an interdisciplinary way.”
Kahn put that concept to work in research highlighted in her book, “One Health and the Politics of Antimicrobial Resistance.” You’ll hear more about Kahn’s research in a future article in this series.
Suffice it to say, her research dismantles the assumptions European regulators made about the presumed link between antibiotic use in livestock and antibiotic resistance in humans, an assumption that continues to drive antibiotic policy there.
More in our antibiotic series:
Part 2: 6 antibiotic myths explained
Part 3: The economics of antibiotic use
“There are a lot of similarities between the evolution of medicine and agriculture over the course of the 20th century,” Kahn explained at the TCFA session. “Both became increasingly specialized, technologically driven and dependent on antibiotics. “
In the case of medical care, the price of care increased dramatically. In the case of agriculture, the price of food decreased, particularly in the United States. “Since World War II, we have been the beneficiaries of the decreasing cost of food, to where we spend less than 10% of our annual disposable income for food. One could argue that our entire consumer economy depends on having inexpensive food,” Kahn said.
Human health
All of that is why antibiotic stewardship — preserving the use of antibiotics for their intended purpose —is gaining urgency in both livestock and human medicine.
“I tell our staff and physicians that antibiotics are unlike any other drug. They’re societal drugs,” Stevenson explained. “If I use an antibiotic in one patient today, it may influence the ability of that drug to effectively treat another patient tomorrow. That’s a point we have to continue to bring home to clinicians.”
Reality paints a less conservative picture of dispensing discretion.
“Estimates are that 30% to 50% of antibiotics prescribed in hospitals are unnecessary or inappropriate; over 50% of antibiotic prescriptions in outpatient clinics are unnecessary,” said Dr. Nate Smith, sharing CDC estimates at the NIAA meeting. He is a physician as well as director and state health officer for the Arkansas Department of Health.
That doesn’t mean unnecessary or inappropriate antibiotic prescriptions are made without thought or care.
“When I talk to physician colleagues, I don’t find any who say, ‘About half of the prescriptions I write for antibiotics are unnecessary,’ ” Smith said. Instead, the ubiquitous nature of such prescriptions has to do with everything from regional culture and relative wealth, to patient expectations, to time.
“Many times, our physicians are working under pressure,” Smith explained. “They need to make decisions quickly, and sometimes with levels of uncertainty.”
For instance, a patient might come in with an upper respiratory tract infection, typically caused by viruses, which are unaffected by antibiotics. But a viral infection can lead to a secondary bacterial infection. Especially if it would be difficult for the patient to make another trip to the office, the physician might go ahead and prescribe an antibiotic.
Unfortunately, this better-safe-than-sorry approach — inappropriate and unnecessary use of antibiotics — can accelerate antibiotic resistance.
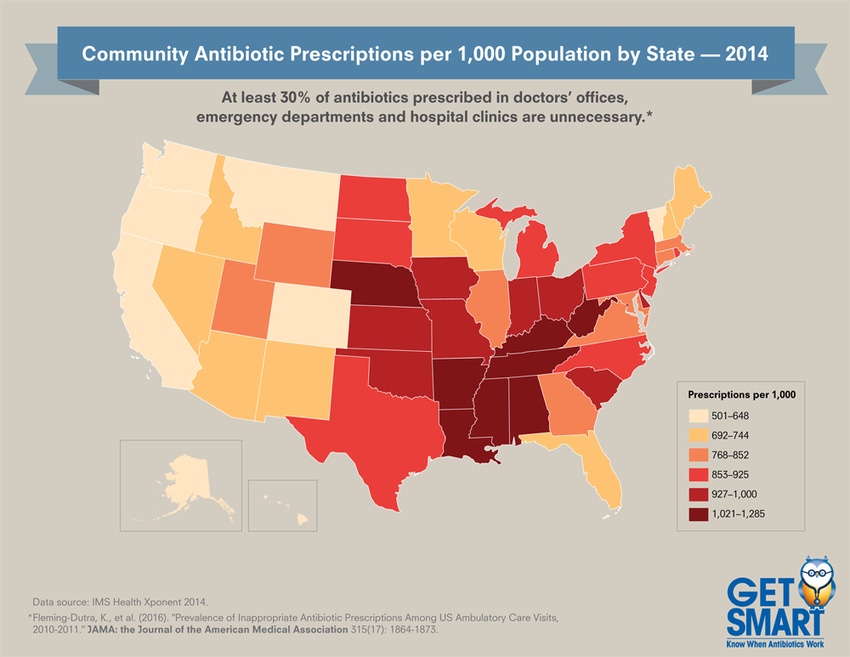
Every resident in some states receives the equivalent of more than one prescription of antibiotics each year (see map). The fewest antibiotic prescriptions per 1,000 people occur in Alaska, at 501 (2014 CDC data) and the most are in West Virginia, at 1,285.
According to CDC, at least 2 million people in the U.S. each year become infected with bacteria that are resistant to antibiotics. At least 23,000 people die each year as a direct result of these infections.
Choosing wisely
“Antimicrobial stewardship includes not only limiting inappropriate use, but also optimizing antimicrobial selection, dosing, route and duration of therapy to maximize clinical cure or prevention of infection — while limiting the unintended consequences, such as the emergence of resistance, adverse drug events and cost,” Stevenson explained.
These elements are central to the multifold antibiotic stewardship programs under way in human medicine at state and national levels.
For instance, the White House released The National Action Plan for Combating Antibiotic-resistant Bacteria (CARB) two years ago, developed by an interagency task force of the same name. Its goals include: slow the emergence of resistant bacteria and prevent the spread of resistant infections; strengthen national One Health surveillance efforts to combat resistance; and accelerate basic and applied research and development for new antibiotics, other therapeutics and vaccines.
Internationally, there are a number of programs. Last year, the United Nation’s General Assembly embraced the World Health Organization’s Global Action Plan on Antimicrobial Resistance. That plan also supports the multi-sector One Health approach to addressing antibiotic resistance.
Keep in mind that the One Health initiative focuses on more than antibiotic resistance. According to its mission statement, One Health seeks to promote, improve, and defend the health and well-being of all species by enhancing cooperation and collaboration between physicians, veterinarians, other scientific health and environmental professionals.
As mentioned previously in this exclusive BEEF magazine series, livestock producers in general, and cattle producers in particular, began addressing antibiotic stewardship years ago. Most recently, the veterinary feed directive implemented Jan. 1 is a governmental effort to regulate stewardship.
“Antibiotics really are the foundation of modern medicine,” Kahn explained. “Without antibiotics, we’re dead in the water when it comes to elective surgeries, cancer chemotherapies and immunosuppressive therapies.
“All of the treatments that we take for granted with modern medicine, we cannot do without antibiotics because the risk of infection is too high … We need to make sure we get this right, because we may not have a second chance.”
Next month: Looking for the antibiotic link between livestock and humans
About the Author(s)
You May Also Like




.png?width=300&auto=webp&quality=80&disable=upscale)
