thumbnail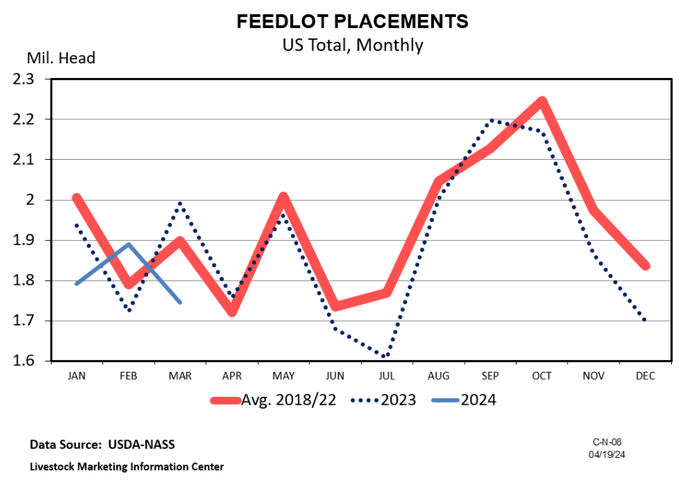
Cattle Market Outlook
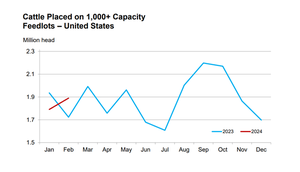
A bullish April cattle on feed reportA bullish April cattle on feed report
The April Cattle on Feed report highlighted a notable decrease in March placements compared to the same period in 2023.
Subscribe to Our Newsletters
BEEF Magazine is the source for beef production, management and market news.



.png?width=700&auto=webp&quality=80&disable=upscale)



.jpg?width=300&auto=webp&quality=80&disable=upscale)