Top Stories

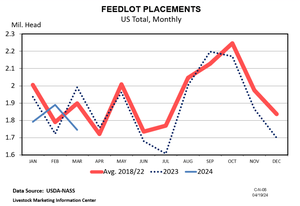
U.S. beef, cattle demand posts early 2024 highs
U.S. beef, cattle demand posts early 2024 highs
Trade deficit continues to widen as beef production declines.
Cash Grain Bids for
Boone, IA
Landus Cooperative (Boone, IA)
corn
ZCK24
$4.32
corn
ZCZ24
$4.41
soybeans
ZSK24
$11.08
soybeans
ZSX24
$11.11
| Contract | Last | Change | High | Low | Open | Last Trade |
|---|---|---|---|---|---|---|
| Jul 24 Corn | 452 | +3.5 | 453.25 | 446.5 | 448.5 | 09:39 PM |
| Jul 24 Oats | 351 | -2.75 | 354.25 | 342.5 | 354 | 09:38 PM |
| May 24 Class III Milk | 18.28 | +0.1 | 18.28 | 18.28 | 18.28 | 10:00 PM |
| Jul 24 Soybean | 1179.75 | -1.75 | 1183.5 | 1166.75 | 1180.25 | 09:38 PM |
| Aug 24 Feeder Cattle | 258.3 | +2 | 258.8 | 254.25 | 256.425 | 06:04 PM |
| May 24 Ethanol Futures | 2.161 | unch — | 2.161 | 2.161 | 0 | 09:38 PM |

Copyright © 2019. All market data is provided by Barchart Solutions.
Futures: at least 10 minute delayed. Information is provided ‘as is’ and solely for informational purposes, not for trading purposes or advice.
To see all exchange delays and terms of use, please see disclaimer.
Farm Business Management
See allLivestock Management
See allVideos
Watch This Week in Agribusiness with Max Armstrong to see what's happening in agriculture each week.
Mike Pearson is talking clean soil, biodiesel and even a look at what’s going on with the apple industry.
Mike Pearson gets you ready for planting season and learns about the tough year for crawfish.
EPA’s snub of renewable fuels, land values, grain bin monitoring, new John Deere equipment, commodity transportation impacts, cover crops and carbon, fuel delivery trailers and supply chains are featured this week.
Sustainability, ag education, crop protection, markets, and more featured this week.
Content Spotlight
Capture uninterrupted immunity by vaccinating calves earlier
Build calf immunity against disease like bovine respiratory disease sooner by vaccinating as early as 30 days of age.


.jpg?width=700&auto=webp&quality=80&disable=upscale)