Banamine Transdermal labeled to offer control of pain with footrot and fever from BRD.
February 1, 2018
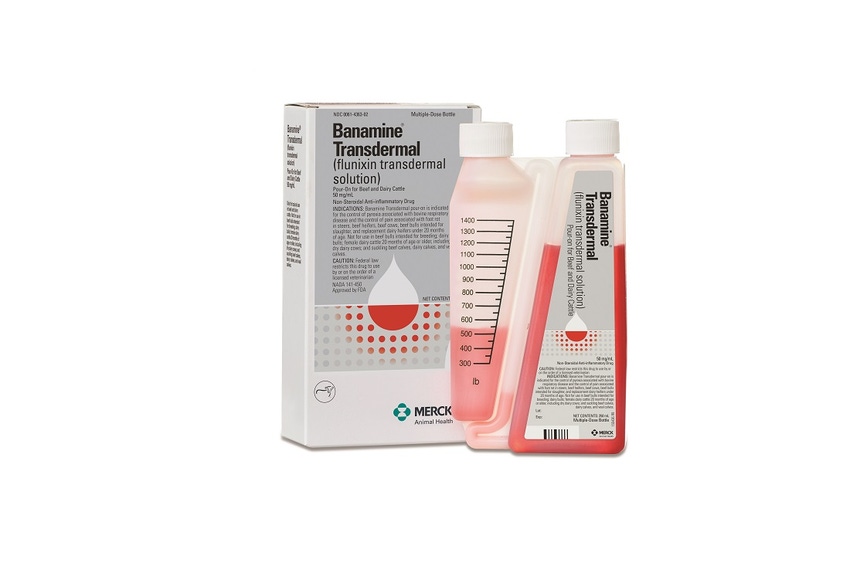
Merck Animal Health today introduced Banamine Transdermal (flunixin transdermal solution) – the first and only US Food & Drug Administration (FDA) approved product for pain control in a food-producing animal.
It is approved for the control of pain associated with foot rot and fever associated with bovine respiratory disease (BRD), and is applied as a pour-on.
The pour-on application eliminates the time-consuming and stressful treatment process associated with intravenous (IV) administration, which is the administration route of previous NSAIDs.
In field studies at four US locations involving animals diagnosed with BRD, 58.3% of cattle treated with Banamine Transdermal had at least a two-degree drop in temperature within six hours of treatment compared to 6.1% for the placebo group. Two studies showed significant results in pain associated with foot rot. These showed that 93.3% to 100% of cattle treated with Banamine Transdermal had improved lameness scores compared with 6.7% and 53.3% for the placebo groups.
A Merck representative said veterinarians and producers are increasingly addressing the relationship between animal well-being and lost performance, as fever and pain can cause cattle to stop eating and drinking.
Banamine Transdermal is a prescription product with pre-calibrated packaging and red-colored solution to help ensure the correct dose is given every time. The unique bottle design makes it simple to apply topically on dry skin in a narrow strip down the animal’s midline from the withers to the tail head.
For information visit BanamineTD.com or contact your veterinarian.
Source: Merck Animal Health
You May Also Like



